Autism spectrum disorder (ASD) is a common neurodevelopmental disorder, affecting about 1% of the population worldwide and for which, currently, there are no effective drugs. Patients often have two core symptoms of ASD, namely, social communication and interaction impairments, and stereotyped repetitive behaviors. At present, mice are commonly used as the animal model of ASD internationally. However, there are substantial differences in brain structure, function and behavior between mice and humans. These differences greatly limit the clinical translation of research done in the mouse models. Many effective drugs tested in neuropsychiatric disease mouse models have no effect on clinical patients which highlights the need to develop alternative animal models. Mutations in the postsynaptic density scaffolding protein, SHANK3, are one of the most common mutations causing autism. Therefore, to better model ASD, we have successfully generated a Shank3 gene-edited canine model using CRISPR/Cas 9 technology.
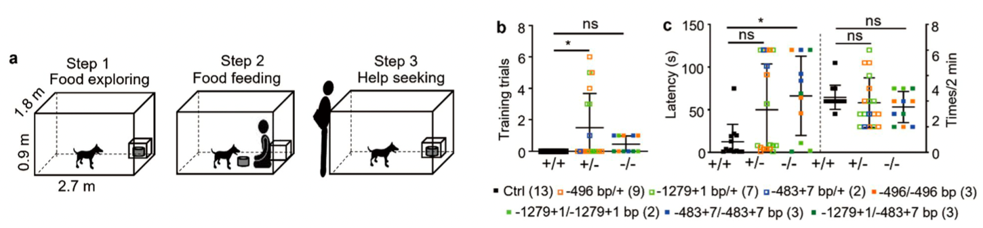
An increased latency of the attention-seeking behaviors is observed in Shank3 mutant dogs as compared with WT dogs, suggesting delayed soliciting behavior in Shank3 mutant dogs.
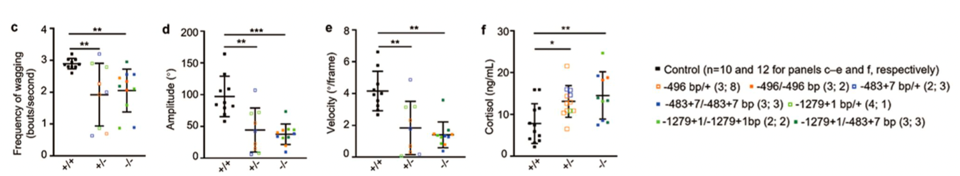
During dog-human interactions, the tail wagging frequency, amplitude and velocity of Shank3 mutant dogs were also significantly reduced, indicating that mutation of Shank3 has significantly impaired social interactions of the dogs with humans.
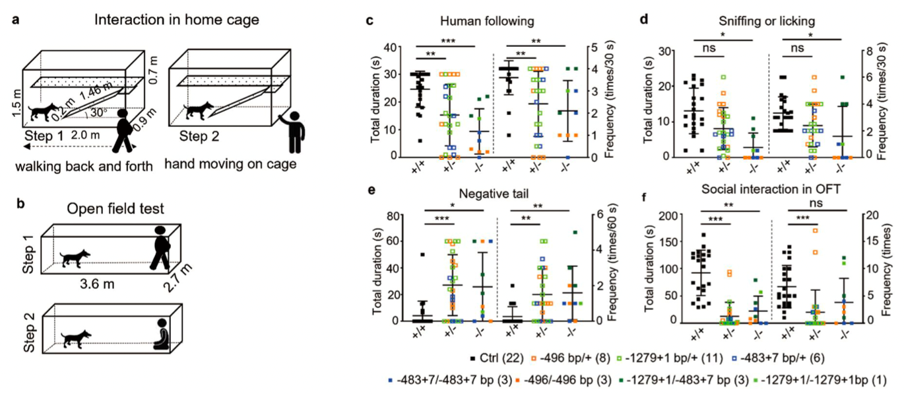
Conducting cage and open field experiments using the dog model, the study found that WT dogs showed great interest in researchers, while Shank3 mutant dogs showed less interest, that is, the duration and frequency of interaction with people were significantly reduced.
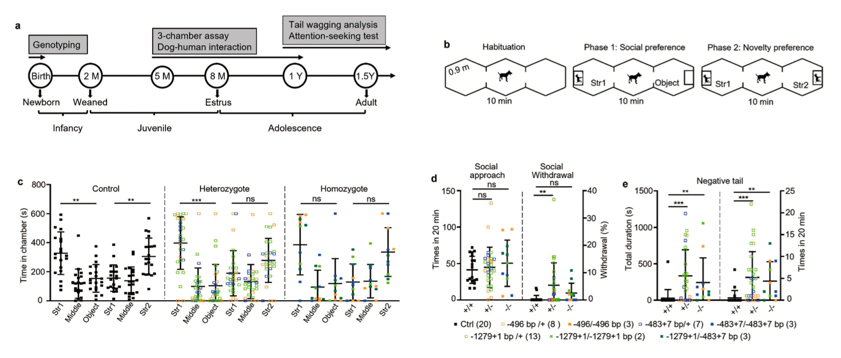
Homozygous Shank3 mutants displayed deficits in both social preference and novelty preference compared with WT dogs. The results showed that although the homozygous dogs had sufficient social motivation, they showed obvious social withdrawal behavior; the duration and frequency of the stressed tail of the heterozygous and homozygous dogs were both higher than that of the WT dogs, reflecting the anxious and fearful inner state of the Shank3 mutant dogs.
Dogs have similar living habits to humans and have rich emotional, social and empathic characteristics, which make them an ideal model for the study of mental disorders.
Shank3 gene-edited dogs exhibited core symptoms of autism such as social deficits (reduced social time, increased social pressure) and stereotyped repetitive behaviors during conspecific (the three-chamber test) and dog–human interactions. In addition, the Shank3 mutant dogs also show comorbid symptoms such as impaired motor function and sleep disorders. In conclusion, the above-mentioned phenotypes of Shank3 mutant dogs recapitulate key phenotypes associated with ASD, which make them an ideal animal model for autism research.
SGene, in collaboration with Zhang Yongqing, a researcher at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, conducted research on autism-related topics. By using behavioral studies, brain imaging, electrophysiology, and molecular detection analysis, in-depth research on the pathogenic mechanisms of autism, as well as efficacy evaluation of relevant drugs and the development of new drugs, were conducted.