The first mutated ALS gene discovered was the superoxide dismutase 1 (SOD1) gene, which encodes a metalloenzyme that converts the highly toxic superoxide anion to molecular oxygen and hydrogen peroxide.
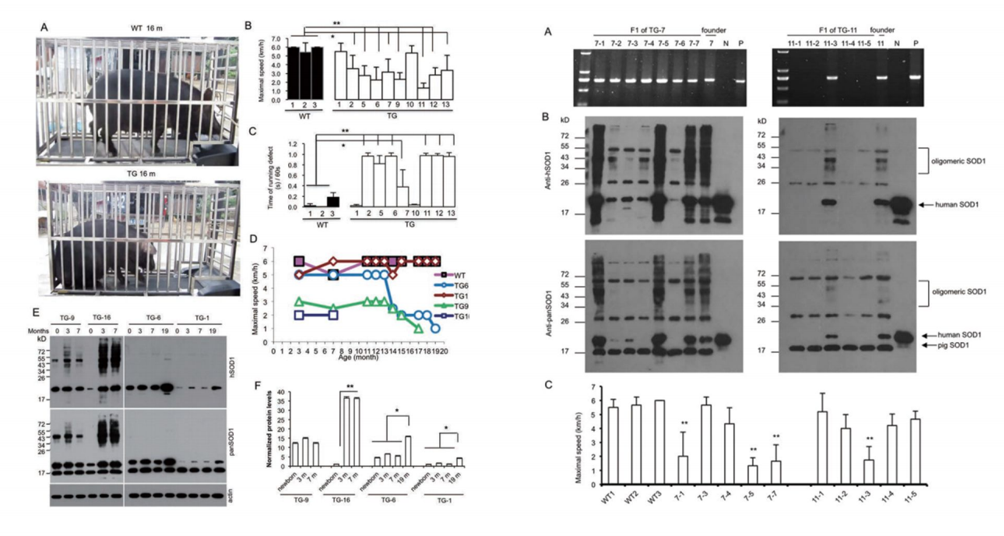
Now over 150 pathogenic mutations in SOD1 have been found in ALS patients. Identification of these mutations has led to a number of animal models expressing ALS-associated SOD1 mutants, providing strong evidence for a gain-of-toxic function of mutant SOD1. We used the SCNT method to generate transgenic pigs that express autosomal-dominant fALS mutants of SOD1 (G93A).
Pig Breed: Tibetan Pig
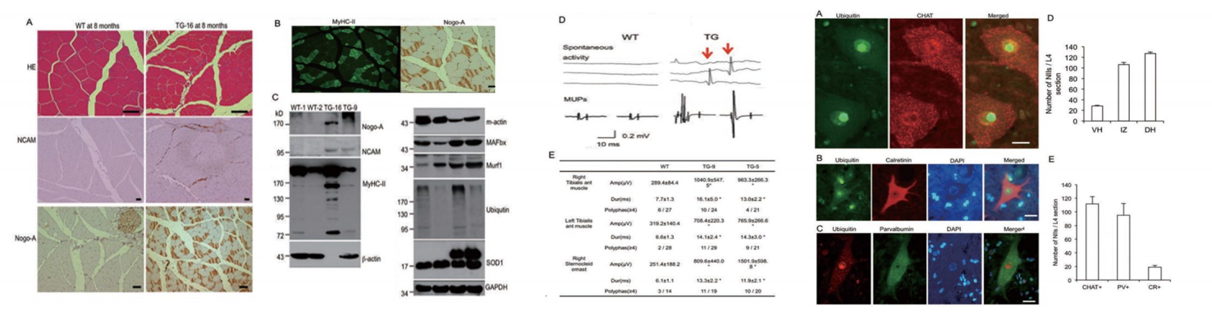
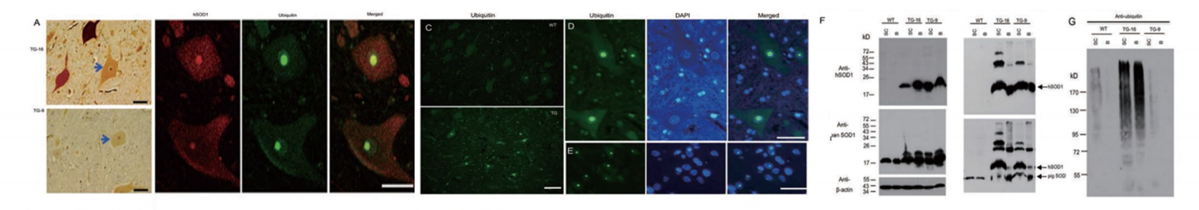
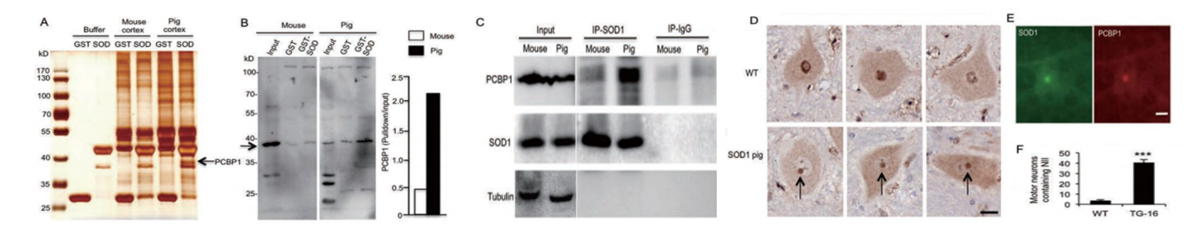
Through nuclear transfer, we have generated transgenic cloned pigs expressing the SOD1-G93A protein mutation found in ALS patients. Pathological and behavioral studies reveal that these transgenic pigs develop hindlimb motor disturbances, associated with motor neuron death, accompanied by astrocyte and microglial proliferation, as well as skeletal muscle atrophy. The hSOD1 transgenic pigs manifest typical disease features of human ALS and unique nuclear inclusions not observed in other ALS animal models. Furthermore, our bred F1 generation hSOD1 transgenic pigs showed inheritability of the mutated gene and phenotype, with offspring also exhibiting motor defects. Hence, our transgenic pigs provide an animal model to study the pathogenesis of ALS and to identify potential targeted therapies.