
电话:+86 13910991951
email:service@sinogene.com.cn

Animal models, as an indispensable tool, have been widely utilized primarily in the studies of life sciences, human medicine, and health. Researchers have gradually discovered that compared to common small animal models, large animal models are more akin to humans in terms of function, structure, and response, and can better simulate human disease characteristics. This similarity is crucial for the evaluation of instrument development, drug assessment, disease treatment, and other aspects.
The gene-edited pig models have become one of the most popular large animal models, due to the many similarities between pigs and humans in terms of anatomical structure, physiological metabolism, and the mechanisms of disease development. In 2021, researchers including Joan K. Lunney from the United States Department of Agriculture collaborated on a review article titled "Importance of the pig as a human biomedical model" published in the journal Science Translational Medicine. This article systematically summarized the current status of pigs as human research models and emphasized the future prospects of genetically edited pig models. It also demonstrated the feasibility and extensive application of genetically edited pig models in various disease areas: these models have been used to study human cancers, cardiovascular diseases, cystic fibrosis (CF), neurodegenerative diseases, and have made xenotransplantation possible. [1]
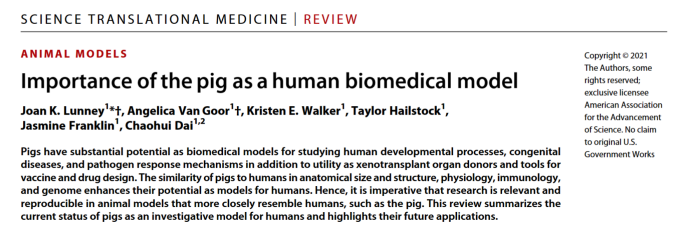
The National Institutes of Health (NIH) in the United States have also expressed support for expanding the application of gene-edited pigs and have established a pig experimental center as part of the Somatic Cell Genome Editing Consortium. In early 2024, the global animal breeding leader, Genus, announced in the academic "The CRISPR Journal" [2] the latest research progress on their genetically edited pigs resistant to blue ear disease, demonstrating a significant advancement from "concept validation" to "commercial." As gene-edited pigs resistant to blue ear disease are expected to become the first gene-edited animal product to enter the consumer market on a large scale in the United States, this news has garnered widespread attention, including from "Science" [3].

Today, a large number of models have been developed and applied in the field of medicine worldwide. Chinese researchers have achieved remarkable success in the field of pig models, Dr. Lai Liangxue's team at the Guangzhou Institute of Biomedicine and Health of the Chinese Academy of Sciences, together with Dr. Li Xiaojiang's research group at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences, successfully established pig for amyotrophic lateral sclerosis (ALS) with the mutated SOD1 gene and for Huntington's disease by knocking in causative gene, accurately mimicking human neurodegenerative diseases. Dr. Lai Liangxue's team also successfully established a genetically edited pig capable of completely secreting insulin. Using a novel conditionally expressed Cas9 gene tool in pig models, they established the first large animal model of primary lung cancer. Additionally, the Xijing Hospital successfully treated severely burned patients using multi-gene-edited pig skin. Furthermore, the first of genetically pigs resistant to porcine reproductive and respiratory syndrome (PRRS) virus, developed independently by the New Hope Group, was born without exogenous DNA integration, and so on. The similarity between pigs and humans makes pig an ideal model for a wide range of diseases. The extensive anatomical and physiological similarities between pigs and humans provide significant advantages for pigs as a model in human medical research. This similarity is not only crucial for uncovering disease mechanisms, identifying drug targets, conducting drug efficacy evaluations, and finding treatment methods, but it also provides researchers with robust conditions for experimental operations, enabling the determination of safe dosage ranges in drug development research and toxicology testing.
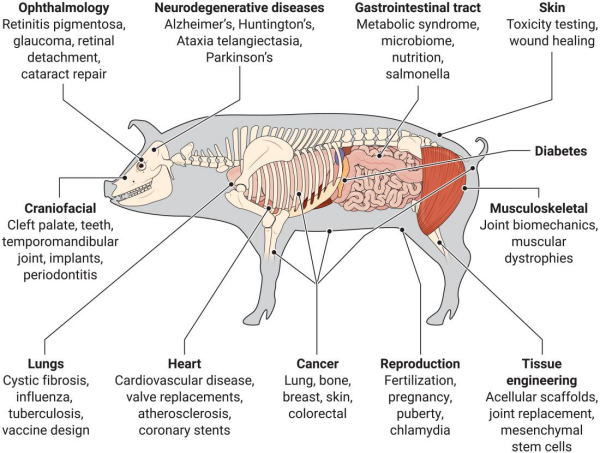
Caption: The pig model can be widely used in human physiology and disease research The human brain shares many similarities with the pig's brain. Like humans, the pig's brain gyrencephalic, and the size of brain is closer to that of the human brain. Additionally, the composition of white matter is similar (with a white-to-gray matter ratio of 60:40), and there are six resting-state networks that are similar to those found in humans. These similarities make the pig model an ideal model for neurodegenerative diseases.
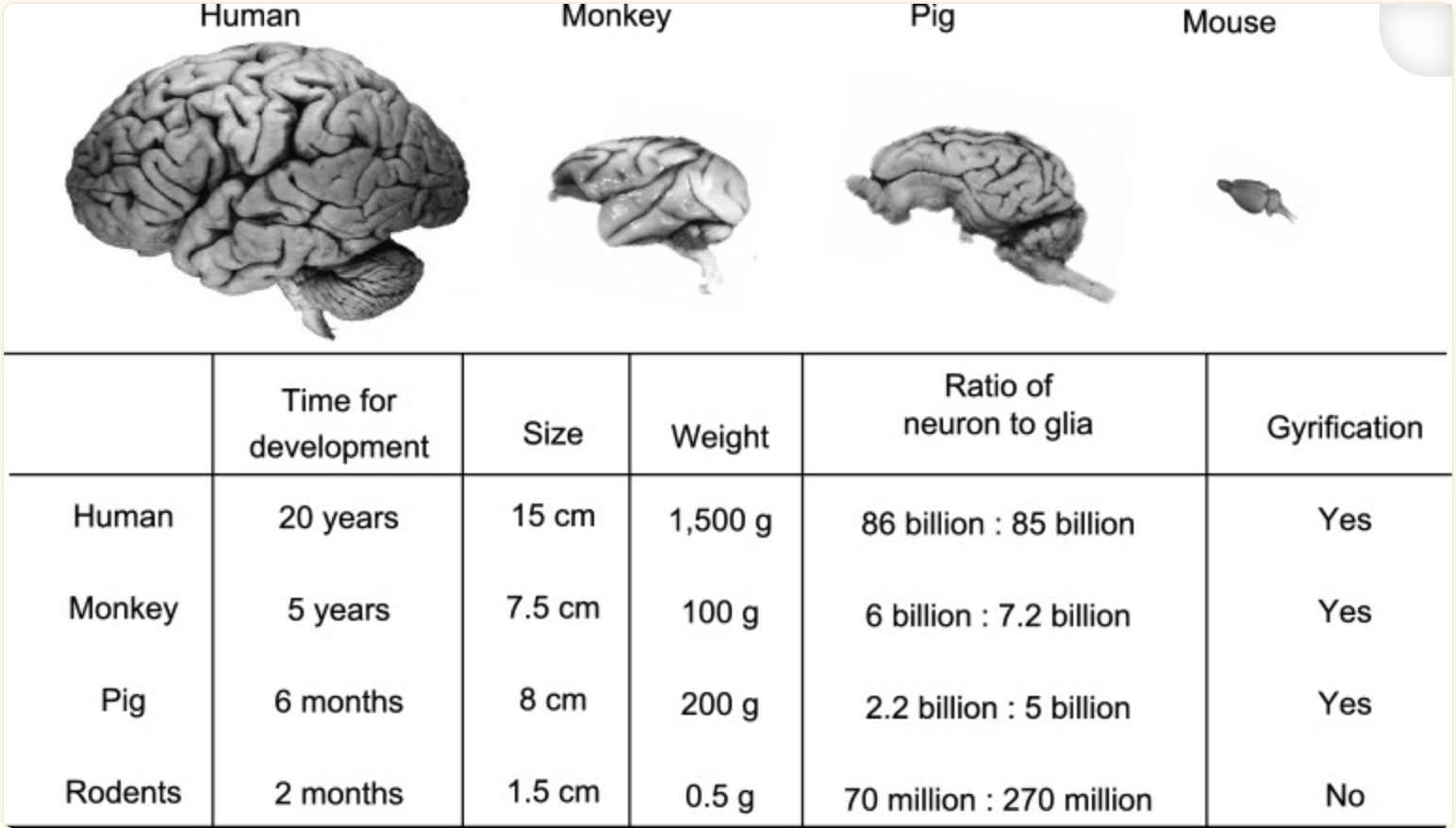
Fig. Major differences in brain size and structures between rodents and large animals[4] Some good examples of pig models:

Abstract:Huntington's disease (HD) is characterized by preferential loss of the medium spiny neurons in the striatum. Using CRISPR/Cas9 and somatic nuclear transfer technology, we established a knockin (KI) pig model of HD that endogenously expresses full-length mutant huntingtin (HTT). By breeding this HD pig model, we have successfully obtained F1 and F2 generation KI pigs. Characterization of founder and F1 KI pigs shows consistent movement, behavioral abnormalities, and early death, which are germline transmittable. More importantly, brains of HD KI pig display striking and selective degeneration of striatal medium spiny neurons. Thus, using a large animal model of HD, we demonstrate for the first time that overt and selective neurodegeneration seen in HD patients can be recapitulated by endogenously expressed mutant proteins in large mammals, a finding that also underscores the importance of using large mammals to investigate the pathogenesis of neurodegenerative diseases and their therapeutics.【5】

Abstract:Mutations in the human copper/zinc superoxide dismutase 1 (hSOD1) gene cause familial amyotrophic lateral sclerosis (ALS). It remains unknown whether large animal models of ALS mimic more pathological events seen in ALS patients via novel mechanisms. Here, we report the generation of transgenic pigs expressing mutant G93A hSOD1 and showing hind limb motor defects, which are germline transmissible, and motor neuron degeneration in dose- and age-dependent manners. Importantly, in the early disease stage, mutant hSOD1 did not form cytoplasmic inclusions, but showed nuclear accumulation and ubiquitinated nuclear aggregates, as seen in some ALS patient brains, but not in transgenic ALS mouse models. Our findings revealed that SOD1 binds PCBP1, a nuclear poly(rC) binding protein, in pig brain, but not in mouse brain, suggesting that the SOD1-PCBP1 interaction accounts for nuclear SOD1 accumulation and that species-specific targets are key to ALS pathology in large mammals and in humans. 【6】

Abstract:Human Waardenburg syndrome 2A (WS2A) is a dominant hearing loss (HL) syndrome caused by mutations in the microphthalmia-associated transcription factor (MITF) gene. In mouse models with MITF mutations, WS2A is transmitted in a recessive pattern, which limits the study of hearing loss (HL) pathology. In the current study, we performed ENU (ethylnitrosourea) mutagenesis that resulted in substituting a conserved lysine with a serine (p. L247S) in the DNA-binding domain of the MITF gene to generate a novel miniature pig model of WS2A. The heterozygous mutant pig (MITF+/L247S) exhibits a dominant form of profound HL and hypopigmentation in skin, hair, and iris, accompanied by degeneration of stria vascularis (SV), fused hair cells, and the absence of endocochlear potential, which indicate the pathology of human WS2A. Besides hypopigmentation and bilateral HL, the homozygous mutant pig (MITFL247S/L247S) and CRISPR/Cas9-mediated MITF bi-allelic knockout pigs both exhibited anophthalmia. Three WS2 patients carrying MITF mutations adjacent to the corresponding region were also identified. The pig models resemble the clinical symptom and molecular pathology of human WS2A patients perfectly, which will provide new clues for better understanding the etiology and development of novel treatment strategies for human HL. 【7】

Abstract:To report the cochlear morphology and electrophysiology of Chinese experimental miniature pigs. Twenty Chinese experimental miniature pigs were used in this study. Auditory brainstem responses (ABR), cochlear endolymphatic potentials (EP), and the potassium concentrations of cochlear endolymph were recorded. Hair cell morphology was examined using electron microscopy. The capsule of cochlea of the miniature pig has three and one-half turns which contains a 39-mm long membranous labyrinth. The organ of Corti in the labyrinth encompasses three rows of outer hair cells and one row of inner hair cells. The stereocilia of the hair cells in the apical turn of the cochlea were significantly longer than those in the basal turn. The vestibular apparatus consists of three semicircular canals and the otolith organs. The average threshold of the ABR was 35-45 dB SPL (n=20) from 4 to 32 kHz. There was no significant difference in the threshold or latency of the ABR between 1-day-old and 30-day-old miniature pigs. The average EP value was 77.3±14 mV (n=9) and the average potassium concentration was 147.1±13 mM (n=5) recorded from the second turn of the cochlea. These studies on the cochlear morphology and electrophysiology of the miniature pigs help to establish the Chinese experimental miniature pig as an animal model for future studies in otology and audiology. 【8】 In conclusion, pigs and humans share many homologies in their genomes. The advantages in body size, physiological metabolism, and disease characteristics make pigs more suitable as models for human diseases. Gene-editing can be used to realize these models. Currently, gene-editing of pigs mainly relies on somatic cell cloning techniques. The development cycle for gene-edited pig models is approximately 10-12 months, but can be expedited to 6-8 months. If reproduction of a generation is desired, an additional cycle of sexual maturity and gestation is required. Reference:
[2] Burger BT, Beaton BP, Campbell MA, et al. Generation of a Commercial-Scale Founder Population of Porcine Reproductive and Respiratory Syndrome Virus Resistant Pigs Using CRISPR-Cas. CRISPR J. 2024 Feb;7(1):12-28.
[4] New pathogenic insights from large animal models of neurodegenerative diseases - PMC (nih.gov)
[6] Species-dependent neuropathology in transgenic SOD1 pigs - PubMed (nih.gov)
Kindly fill out this short message and we will contact you in 24hrs.